Task2.1 MCCs production through anaerobic fermentation
Task2.1.1 Operating conditions screening tests at microcosm scale
Firstly, the feasibility of producing MCCs from different actual substrates will be studied at microcosm scale (0.1-1L), using CRFSS hydrolysate (generated in Task1.3), spent yeast (generated at the TGs separation step, Task2.2.3) and glycerol (generated in the production of biodiesel 2, Task3.2). To this aim, previous reported inoculum, culture conditions (pH 7, 37°C and 150 rpm) and analytical procedures will be used. Regarding the last, µ-GC and HPLC-rid/GC-fid will be used for monitoring biogas and carboxylic acids compositions, respectively. In this stage also the co-fermentation of two or more substrates will be studied with another set of experiments. Secondly, kinetic studies will be performed using the substrates (and/or mixture of it) that resulted to produce MCCs (result of previous experiment). Such kinetic test, allowing to optimize the substrate concentration condition, will be carried out using the same microcosm scale and procedures previously described.
Task2.1.2 MCCs production in bench scale bioreactor
MCCs will be produced using 3-30 L bioreactor with automatic pH control. This will allow to: 1) obtain more representative results for scaling up considerations, 2) produce higher amounts (200 g) required for the catalytic hydrogenation tests and 3) generate 30 L of actual MCCs-rich broth required for assessing the recovery with a BPM-ED. To this aim, substrate/s will be anaerobically fermented at the optimal conditions previously found in Task2.1.1. At the end of each batch, the solids will be separated from the broth by means of filtration and centrifugation. The liquid will be stock until used for recovering the MCCs.
Task2.1.3 Separation of highly concentrated MCCs
Firstly, MCCs will be separated by adding inorganic acid (HCl or H2SO4) as recently reported by Martinez et al. This will allow to obtain highly concentrated MCCs that can be used in the catalytic reductive esterification tests of Task3.3.
Secondly, the application of BPM-ED will be assessed as it would allow increasing the recovery of MCCs (respect to the separation with inorganic acid addition) but also it would allow to recover the Na+ (originally added during the anaerobic fermentation for maintaining the pH level at 7). To this aim, a commercial bench scale ED unit (25 L/h of treatment capacity) will be used. The unit allows assessing two or three compartment configurations: the first obtains cation-rich and acid-rich streams, whereas the second obtains cationic-rich, diluted and acid-rich streams.
First experiments will be performed by using a laboratory prepared solution (organic acids, Cl-, PO4x-, SO4x- and Na+) that simulates the actual MCCs-rich broth. Such tests will allow to optimize the operating conditions and to develop operating procedures of sequential concentration batch (for simulating feed and bleed process). Second experiments will be carried using the actual MCCs-rich broth at the best preciously founded conditions in order to assess the membrane fouling with the complex matrix as well as the actual recovery and energy yields.
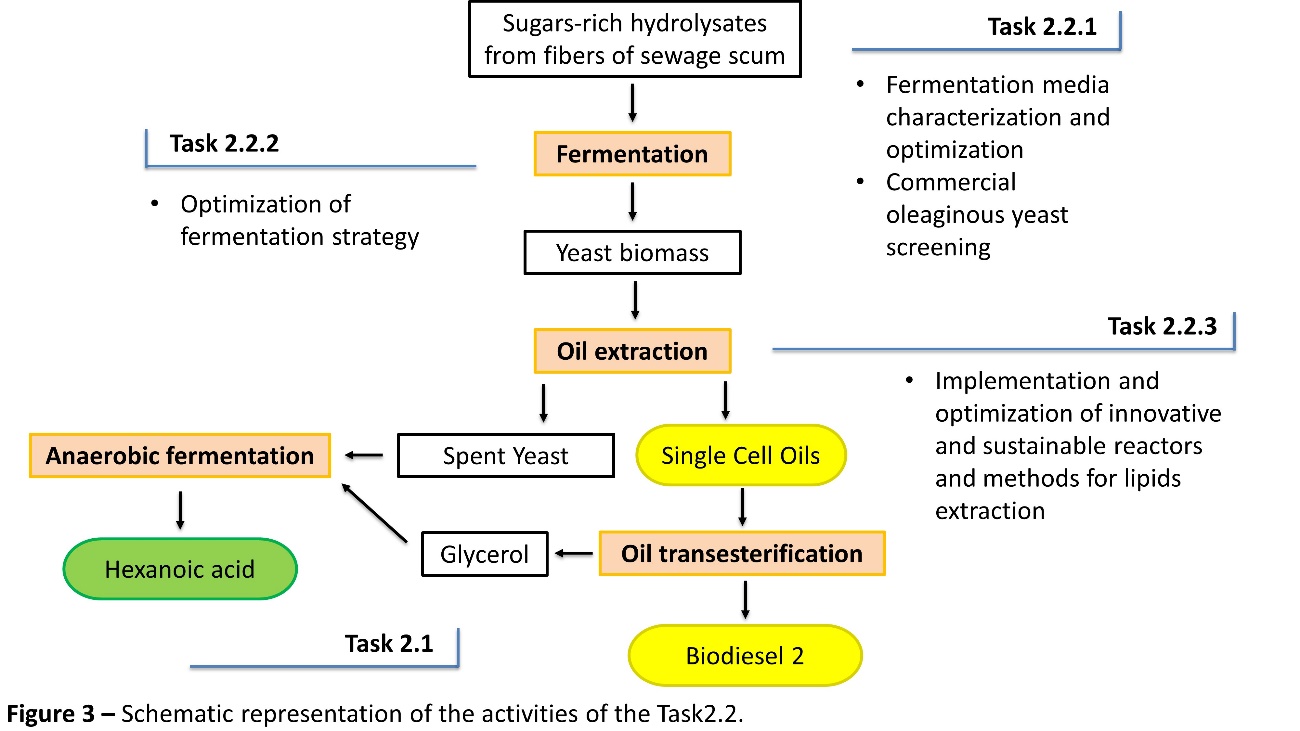
Task2.2 Yeast fermentation
OYs have a favorable lipid profile for the production of biodiesel, due to their high percentage of oleic acid, and they are a potential solution for all the sustainability issues related with first-generation biodiesel production. However, in the perspective of a potential industrial scale-up, some innovation and improved technical solutions are still required related to both upstream and downstream process steps, especially when residual biomass and agro-industrial wastes are use as starting raw material. For the upstream section, an issue is often related to the complete exploitation of the carbon source, since very high concentration of sugars required long process time or does not allow the complete bioconversion of sugars to give TGs. Moreover, the lipogenic performance of oleaginous yeasts is strictly related to the chemical composition of the culture medium, especially when complex substrates, such as SS, are used as carbon source for the oil accumulation. An important challenge for SCOs production is represented by the technology adopted for lipids extraction, namely for the downstream section. In the literature, various physico-chemical disruption methods of the yeast cell wall are reported, characterized by different oil extraction efficiency and effects on the composition of fatty acids. The key goals of the ReFil project for enhancing microbial lipids yield from SS hydrolysates for biodiesel production are the following:
Task2.2.1. Yeast screening and fermentation media formulation for lipids accumulation
The optimization of fermentation broth, namely SS hydrolysates, aims to define the optimal concentration of sugars (glucose, xylose, arabinose), nitrogen source (organic or inorganic) and inorganic nutrients in order to maximize the yeast growth and the lipid accumulation. The optimization of the chemical composition of fermentation broth will be performed by the use of chemometric approaches.
Moreover, the selection of robust and efficient commercially available yeast species will be accomplished by means of lab-scale screening. The ability to use complex carbon source, such as SS hydrolysates, and the ability to grow in the presence of inhibitor compounds such as furan derivatives and phenolics is strain dependent. For this reason, a strategic preliminary approach is the investigation of the fermentation performance of different yeast species, such as Lipomyces starkeyi, Cryptococcus curvatus, Rhodosporidium toruloides and Trichosporon oleaginosus, that are characterized by a good resistance to toxic compounds and the ability to use various organic molecules as carbon source.
Task2.2.2. Optimization of lipids accumulation
The fermentation strategy (batch or fed-batch) will be optimized in order to implement a two-stage process: the first stage aiming to maximize the production of yeast biomass and the second one aiming to maximize the lipogenesis by modulating the yeast inoculum, the carbon-to-nitrogen ratio in the culture medium and the frequency of the fresh feed.
Task2.2.3. Recovery of new generation oil
The implementation of innovative and sustainable reactors and methods for lipids extraction from yeast cells will be based on the synergistic combinations of microwave-assisted heating, mild electric field application, and green organic solvents, such as 2-methyltetrahydrofuran, methyl ethyl ketone, ethyl acetate and bioethanol. Moreover, in the perspective of integrated biorefinery processes and circular economy, the chemical characterization and valorization of the spent yeast biomass (waste) obtained from lipids extraction is proposed as carbon and nitrogen source in the anaerobic fermentation to give HexAc (Task2.1) or in the anaerobic digestion to give methane (Task2.3).
The main aim of Task2.2 is to contribute to the advancement of knowledge on new generation biodiesel production from fibers hydrolysates of SS in the perspective of a more economically and environmentally sustainable biorefinery scheme.
Task2.3. Organic leftover valorization through methane-rich biogas production
The organic leftover eventually generated in the SS valorization scheme (e.g. solids separated after MCCs production) will be tested for methane-rich biogas production. Specifically, biomethane potential will be assessed at 100 mL microcosm, using commercial inoculums and at well-stablished operating conditions, i.e. 45-55°C, ISR 1-2 and TS<6%.




